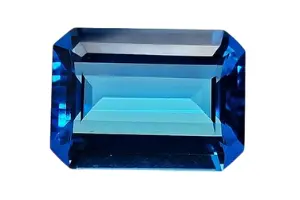
Gemfame.com is a prominent online provider of genuine quality Precious and Semiprecious stones with valid trust at an affordable price for decades. It is an ultimate destination with a range of extensive multinational collections of Gemstones to choose from and procures with the utmost satisfaction of the Customers. It is also a well-known outstanding service provider with full care, coordination, and expert consultation of the desired products of the Customers to get the best value for money.
best selling gemstones
Your one-stop destination shop for Best Selling Gemstones, marvels, and choicest gems with exceptional quality at a great price, which world’s untold treasures bring your life delight.
exclusive gemstones
If you are looking for something exclusively different and special, you are in the right place. Step into our most reliable online shop. and find that perfect jewel stone to suit your every need.
Popular gemstones
The best-trusted place for buying precious gemstones for everything you need with the most reliable source. We provide you with a range of precious semi-precious popular gemstone collections with matchless support.
Earrings / Pendant Loose Gemstone set
Brooch Loose Gemstone set
Earrings / Pendant Loose Gemstone set
Earrings / Pendant Loose Gemstone set
Pendant Loose Gemstone set
Earrings / Pendant Loose Gemstone set
Earrings / Pendant Loose Gemstone set
Necklace / Earrings / Ring Loose Gemstone set
Necklace / Earrings / Ring Loose Gemstone set
Earrings / Pendant Loose Gemstone set
Why Choose GemFame

Huge Collection

Rare Collections

Certified Gemstones

Transparent Pricing
Easy Returns